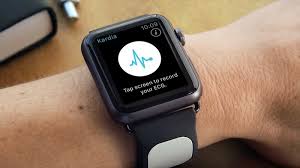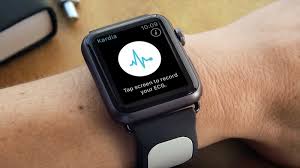
Users of Apple Watch would now be able to inconspicuously capture gather their personal electrocardiogram (EKG) anytime, anywhere so that they are enabled to identify detect normal sinus heart rhythms and atrial fibrillation (AFib), very fast, which are the most common heart arrhythmia, as the FDA now has cleared KardiaBand in the U.S, announced AliveCor.
With the help of just a touch of the integrated sensor, EKG can be recorded by KardiaBand in just 300 seconds. This is the first medical device accessory for Apple Watch that has been cleared by the FDA. The Apple watch display would flash the results from the Kardia App.
SmartRhythm is a new feature in the Kardia app which is also being launched by AliveCor for Apple Watch. A continuous evaluation to measure the relation between heart activity and physical activity owould be possible by SmartRhythm with the usage of artificial intelligence together with data from the Apple Watch's heart rate and activity sensors. In case there is an anomaly in the synchronization of the heart rate and physical activity and detected by SmartRhythm, Apple Watch users would automatically and immediately be alerted to conduct an EKG with the help of KardiaBand, or with KardiaMobile, which is a portable EKG reader.
"KardiaBand paired with SmartRhythm technology will be life-changing for people who are serious about heart health," said Vic Gundotra, CEO, AliveCor. "These capabilities will allow people to easily and discreetly check their heart rhythms when they may be abnormal, capturing essential information to help doctors identify the issue and inform a clear path of care to help manage AFib, a leading cause of stroke, and other serious conditions."
One of the leading causes of stroke and amongst the most common of heart arrhythmia is Atrial Fibrillation (AFib). At least a quarter of world’s population over the age of 40 run the risk of developing AFib which impacts over 30 million people globally every year. there are millions of people in the world who continue to live with AFib knowingly. Yet, in cases where AFib is detected and treated properly, it can have the impact of prevention of two out of three strokes.
"This is a paradigm shift for cardiac care as well as an important advance in healthcare," said Dr. Ronald P. Karlsberg, Clinical Professor of Medicine, Cedars Sinai Heart Institute "Today, EKGs are available only in offices and hospitals, using complex equipment, and usually only after a life threatening event, for example a stroke. With an EKG device on the wrist, AFib can be detected wherever the patient is, 24 hours a day. In randomized research trials, KardiaMobile, the first AliveCor EKG device, proved to be superior to routine care provided by physicians. Today, KardiaBand is a giant leap in personalized health care."
The price of KardiaBand starts at $199 and has to be jointly bought with a AliveCor's Premium service for $99 a year.
(Sourcec:www.prnewswire.com)
With the help of just a touch of the integrated sensor, EKG can be recorded by KardiaBand in just 300 seconds. This is the first medical device accessory for Apple Watch that has been cleared by the FDA. The Apple watch display would flash the results from the Kardia App.
SmartRhythm is a new feature in the Kardia app which is also being launched by AliveCor for Apple Watch. A continuous evaluation to measure the relation between heart activity and physical activity owould be possible by SmartRhythm with the usage of artificial intelligence together with data from the Apple Watch's heart rate and activity sensors. In case there is an anomaly in the synchronization of the heart rate and physical activity and detected by SmartRhythm, Apple Watch users would automatically and immediately be alerted to conduct an EKG with the help of KardiaBand, or with KardiaMobile, which is a portable EKG reader.
"KardiaBand paired with SmartRhythm technology will be life-changing for people who are serious about heart health," said Vic Gundotra, CEO, AliveCor. "These capabilities will allow people to easily and discreetly check their heart rhythms when they may be abnormal, capturing essential information to help doctors identify the issue and inform a clear path of care to help manage AFib, a leading cause of stroke, and other serious conditions."
One of the leading causes of stroke and amongst the most common of heart arrhythmia is Atrial Fibrillation (AFib). At least a quarter of world’s population over the age of 40 run the risk of developing AFib which impacts over 30 million people globally every year. there are millions of people in the world who continue to live with AFib knowingly. Yet, in cases where AFib is detected and treated properly, it can have the impact of prevention of two out of three strokes.
"This is a paradigm shift for cardiac care as well as an important advance in healthcare," said Dr. Ronald P. Karlsberg, Clinical Professor of Medicine, Cedars Sinai Heart Institute "Today, EKGs are available only in offices and hospitals, using complex equipment, and usually only after a life threatening event, for example a stroke. With an EKG device on the wrist, AFib can be detected wherever the patient is, 24 hours a day. In randomized research trials, KardiaMobile, the first AliveCor EKG device, proved to be superior to routine care provided by physicians. Today, KardiaBand is a giant leap in personalized health care."
The price of KardiaBand starts at $199 and has to be jointly bought with a AliveCor's Premium service for $99 a year.
(Sourcec:www.prnewswire.com)