The battery industry has not seen a revolutionary change in the basic way a battery operates until now. Since long have researchers looked for ways to cut costs, store energy and to do so they have typically had to rely on precious metals such as platinum to act as catalysts. Although the use of precious have naturally hiked the cost of fuel cells, they have also made them more efficient in their working.
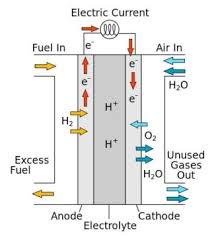
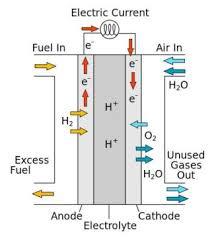
A fuel cell converts chemical energy into electricity by reacting hydrogen and oxygen at two different electrodes. A catalyst makes the reaction more efficient.
In a study published on the 15th of July 2015, a team of chemists from the University of Wisconsin-Madison have had a remarkable breakthrough: they have found a new approach that does not need any metal to acts as a catalyst, instead it uses certain kinds of molecules to act as one. Although this route had been explored in the past, the efficiency of the batteries have been a question mark.
Professor Shannon Stahl from the University of Wisconsin-Madison along with James Gerken, a lab scientist, took inspiration from their previous work with catalysts that make use of oxygen molecules for the chemical industry. The similarity of the reaction of aerobic oxidation and the reaction of oxygen molecules in fuel cells dawned upon them and they wondered if a similar approach could work with fuel cells as well.
They then created a new catalyst which is a mixture of nitroxyls molecules and those from nitrogen oxide. One reacts well with the electrode while the other reacts efficiently with the oxygen.
"While this catalyst combination has been used previously in aerobic oxidations, we didn't know if it would be a good fuel cell catalyst. It turns out that it is the most effective molecular catalyst system ever reported," said Stahl.
Since their approach involved chemical reactions between gases, liquids and solids, it was quite a feat to design a prototype. Gerken spent months studying and optimizing each component of the setup so that the model they developed closely resembled what they had envisaged.
"This work shows for the first time that molecular catalysts can approach the efficiency of platinum. And the advantage of molecules is that you can continue to modify their structure to climb further up the mountain to achieve even better efficiency," said Gerken.
The ground breaking work was funded by the U.S. Department of Energy through the Center for Molecular Electrocatalysis. Both researchers, Gerken and Stahl credit the center for promoting cross-pollination among various chemistry disciplines so as to open new vistas that will provide future advancements in this area.
References:
http://news.wisc.edu/23894
A fuel cell converts chemical energy into electricity by reacting hydrogen and oxygen at two different electrodes. A catalyst makes the reaction more efficient.
In a study published on the 15th of July 2015, a team of chemists from the University of Wisconsin-Madison have had a remarkable breakthrough: they have found a new approach that does not need any metal to acts as a catalyst, instead it uses certain kinds of molecules to act as one. Although this route had been explored in the past, the efficiency of the batteries have been a question mark.
Professor Shannon Stahl from the University of Wisconsin-Madison along with James Gerken, a lab scientist, took inspiration from their previous work with catalysts that make use of oxygen molecules for the chemical industry. The similarity of the reaction of aerobic oxidation and the reaction of oxygen molecules in fuel cells dawned upon them and they wondered if a similar approach could work with fuel cells as well.
They then created a new catalyst which is a mixture of nitroxyls molecules and those from nitrogen oxide. One reacts well with the electrode while the other reacts efficiently with the oxygen.
"While this catalyst combination has been used previously in aerobic oxidations, we didn't know if it would be a good fuel cell catalyst. It turns out that it is the most effective molecular catalyst system ever reported," said Stahl.
Since their approach involved chemical reactions between gases, liquids and solids, it was quite a feat to design a prototype. Gerken spent months studying and optimizing each component of the setup so that the model they developed closely resembled what they had envisaged.
"This work shows for the first time that molecular catalysts can approach the efficiency of platinum. And the advantage of molecules is that you can continue to modify their structure to climb further up the mountain to achieve even better efficiency," said Gerken.
The ground breaking work was funded by the U.S. Department of Energy through the Center for Molecular Electrocatalysis. Both researchers, Gerken and Stahl credit the center for promoting cross-pollination among various chemistry disciplines so as to open new vistas that will provide future advancements in this area.
References:
http://news.wisc.edu/23894